What is Dolomite?
• Chemistry: CaMg(CO3)2,
Calcium Magnesium Carbonate
• Class: Carbonates
• Group: Dolomite
• Uses: in some cement, as a source of
magnesium and as mineral specimens.
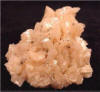
• Specimens
Dolomite, which is named for the French
mineralogist Deodat de Dolomieu, is a
common sedimentary rock-forming mineral
that can be found in massive beds
several hundred feet thick. They are
found all over the world and are quite
common in sedimentary rock sequences.
These rocks are called appropriately
enough dolomite or dolomitic limestone.
Disputes have arisen as to how these
dolomite beds formed and the debate has
been called the "Dolomite Problem".
Dolomite at present time does not form
on the surface of the earth; yet massive
layers of dolomite can be found in
ancient rocks. That is quite a problem
for sedimentologists who see sandstones,
shales and lime stones formed today
almost before their eyes. Why no
dolomite? Well there are no good simple
answers, but it appears that dolomite
rock is one of the few sedimentary rocks
that undergoes a significant
mineralogical change after it is
deposited. They are originally deposited
as calcite/aragonite rich lime stones,
but during a process call diagenesis the
calcite and/or aragonite is altered to
dolomite. The process is not
metamorphism, but something just short
of that. Magnesium rich ground waters
that have a significant amount of
salinity are probably crucial and warm,
tropical near ocean environments are
probably the best source of dolomite
formation.
Dolomite in addition to the sedimentary
beds is also found in metamorphic
marbles, hydrothermal veins and
replacement deposits. Except in its
pink, curved crystal habit dolomite is
hard to distinguish from its second
cousin, calcite. But calcite is far more
common and effervesces easily when acid
is applied to it. But this is not the
case with dolomite which only weakly
bubbles with acid and only when the acid
is warm or the dolomite is powdered.
Dolomite is also slightly harder, denser
and never forms scalenohedrons
(calcite's most typical habit).
Dolomite differs from calcite, CaCO3,
in the addition of magnesium ions to
make the formula, CaMg(CO3)2.
The magnesium ions are not the same size
as calcium and the two ions seem
incompatible in the same layer. In
calcite the structure is composed of
alternating layers of carbonate ions, CO3, and
calcium ions. In dolomite, the
magnesiums occupy one layer by
themselves followed by a carbonate layer
which is followed by an exclusively
calcite layer and so forth. Why the
alternating layers? It is probably the
significant size difference between
calcium and magnesium and it is more
stable to group the differing sized ions
into same sized layers. Other carbonate
minerals that have this alternating
layered structure belong to the Dolomite
Group. Dolomite is the principle member
of the Dolomite Group of minerals which
includes ankerite, the only other
somewhat common member.
Dolomite forms rhombohedrons as its
typical crystal habit. But for some
reason, possibly twinning, some crystals
curve into saddle-shaped crystals. These
crystals represent a unique crystal
habit that is well known as classical
dolomite. Not all crystals of dolomite
are curved and some impressive specimens
show well formed, sharp rhombohedrons.
The luster of dolomite is unique as well
and is probably the best illustration of
a pearly luster. The pearl-like effect
is best seen on the curved crystals as a
sheen of light can sweep across the
curved surface. Dolomite can be several
different colors, but colorless and
white are very common. However it is
dolomite's pink color that sets another
unique characteristic for dolomite.
Crystals of dolomite are well known for
their typical beautiful pink color,
pearly luster and unusual crystal habit
and it is these clusters that make very
attractive specimens.
Dolomite is after calcite the second
most important and abundant of the
carbonate minerals. Chemically and
structurally it may be regarded as
calcite with half the calcium ions
replaced by magnesium. Iron or manganese
may substitute for magnesium in
dolomite, forming isostructural series
with ankerite and Kutnahorite.
The crystal structure, hexagonal-rhombohedral,
is similar to that of calcite, with
alternate layers of calcium ions totally
replaced by magnesium. This ordered
arrangement of cations slightly impairs
the overall symmetry of the structure
but is essential to the stability of the
mineral. Hardness is 4.5-5, specific
gravity 2.85, luster vitreous to pearly,
color ranges from colorless to white
with green, brown, or pink tints, and
cleavage is perfect in three directions.
Like calcite, dolomite occurs in
virtually all geologic settings: in
igneous rocks as carbonatite, in
metamorphic rocks as marble, and in
hydrothermal deposits. Also like
calcite, the most abundant occurrences
are in sedimentary rocks; rock composed
primarily of dolomite is sometimes
referred to as dolostone.
There is uncertainty as to the cause of
its formation, as vast deposits are
present in ancient rock, but it is very
rarely found being produced in modern
environments. This is referred to as the
"Dolomite Problem". Dolomite accounts
for about 10% of all sedimentary rock,
including much that would have been
produced near the surface of the Earth.
However, experiments have only been able
to synthesize dolomite under the high
temperatures and pressures present in
deeper layers.
The Present Scenario About Dolomite
Dolomite at present time, does not form
on the surface of the earth; yet massive
layers of dolomite can be found in
ancient rocks. That is quite a problem
for sedimentologists who see sandstones,
shales and limestones formed today
almost before their eyes.
DOLOMITE is a double carbonate of calium
and magnesium, CaCO3, MgCO3. The mineral
was first identified by Count Dolomien
in 1791 and named after its discoverer.
It is of sedimentary origin and is
supposed to have been formed due to
chemical action of sea-water containing
high percentage of magnesia, on limestone.
Theoretically, Dolomite Contains
| CaCO3 |
54.35% |
 |
| MgCO3 |
45.65% |
In Other Words, Dolomite Contains
| CaO |
30.4% |
 |
| MgO |
21.7% |
| CO2 |
47.9% |
In nature, considerable variations in
the composition of dolomite relating to
lime and magnesia percentages are found.
When the percentage of CaCO3 increases
by 10% or more over the theoretical
composition, the mineral is termed 'calcitic
dolomite', 'high-calcium dolomite' or
'lime-dolomite'. With the decrease in
percentage of MgCO3, it is called 'dolomitic
limestone'. With the variations of MgCO3
between 5 to 10%, it is called 'magnesian
limestone', and upto 5% MgCO3 or less it
is taken to be limestone for all
purposes in trade and commercial
parlance.
Dolomite usually contains impurities,
chiefly silica, alumina and iron oxide.
For commercial purposes, the percentage
of combined impurities should not go
beyond 7% above which, it becomes
unsuitable for industrial use. It is
then used only for road ballasts,
building stones, flooring chips etc.
| Hardness |
3.5-4 |
| Associated |
include calcite sulfide ore
minerals fluorite barite quartz
and occasionally with gold |
| Minerals Chemical/Typical
composition |
white |
| Color |
often pink or pinkish and
can be colorless, white, yellow,
gray or even brown or black when
iron is present in the crystal |
| Characteristics |
Unlike calcite, effervesces
weakly with warm acid or when
first powdered with cold HCl |
| Luster |
pearly to vitreous to dull |
| Field Indicators |
typical pink color, crystal
habit, hardness, slow reaction
to acid, density and luster |
Dolomite Physical Characteristics
| Hardness |
3.5-4 |
| Specific gravity |
2.86 (average) |
| Cleavage |
|
| Color |
Often pink or pinkish and can be colorless, white, yellow, gray or even brown or
black when iron present in the crystal. |
| Density |
|
| Diaphaniety |
|
| Fracture |
Conchoidal |
| Crystal Habits |
Include saddle shaped rhombohedral twins and simple rhombs some with slightly
curved faces, also prismatic, massive, granular and rock forming. Never found in
scalenohedrons. |
| Luminescence |
|
| Luster |
Pearly to vitreous to dull |
| Streak |
White |
| Synonym |
|
| Transparency |
Crystals are transparent to translucent |
| Crystal System |
Trigonal; bar 3 |
| Cleavage |
Perfect in three directions forming rhombohedrons. |
| Other Characteristics |
Unlike calcite, effervesces weakly with warm acid or when first powdered with
cold HCl. |
| Associated Minerals |
Include calcite, sulfide ore minerals, fluorite, barite, quartz and occasionally
with gold |
| Notable Occurrences |
Many localities throughout the world, but well known from sites in Midwestern
quarries of the USA; Ontario, Canada; Switzerland; Pamplona, Spain and in Mexico
|
| Best Field |
Typical pink color, crystal habit, hardness, slow reaction to acid, density and
luster |
Dolomite Chemical Analysis
| Chemical Analysis |
% |
| SiO2 |
------ |
| Al2O3 |
0.04 |
| Fe2O3 |
0.024 |
| TiO2 |
N.D |
| CaO |
32.218 |
| MgO |
20.179 |
| Na2O |
------ |
| K2O |
------ |
| Insoluble |
0.094 |
| Na2CO3 |
------ |
| Loss |
47.33 |
| Total |
99.885 |
Dolomite Habits
Crystalline - Coarse - Occurs as
well-formed coarse sized crystals.
Massive - Uniformly indistinguishable
crystals forming large masses., Blocky - Rhombohedral - Crystal shape resemb les
rhomohedrons.
Associated Minerals include albite,
anatase, calcite, chlorite group,
fluorapatite, fluorite, galena,
gmelinite, marcasite, molybdenite,
pyrite, quartz, rutile, siderite and
sphalerite
Crystal habits include saddle shaped
rhombohedral twins and simple rhombs
some with slightly curved faces, also
prismatic, massive, granular and rock
forming. Streak is white.
The Dolomite Group of Minerals
The Dolomite Group is composed of
minerals with an unusual trigonal bar 3
symmetry. The general formula of this
group is AB(CO3)2, where A can be either
calcium, barium and/or strontium and the
B can be either iron, magnesium, zinc
and/or manganese.
The structure of the Dolomite Group is
taken from the Calcite Group structure.
The Calcite Group structure is layered
with alternating carbonate layers and
metal ion layers. The structure of the
Dolomite Group minerals is layered in
such a way that the A metal ions occupy
one layer which is followed by a
carbonate layer which is followed by the
B metal ion layer followed by another
carbonate (CO3) layer, etc. The layering
looks like this:
|A|CO3|B|CO3|A|CO3|B|CO3|A|... This
ordered layering of different or
nonequivalent ions causes a loss of the
two fold rotational axes and mirror
planes that are present in the Calcite
Group structure. Dolomite's symmetry
class is bar 3 whereas the Calcite
Group's symmetry class is bar 3 2/m. The
loss of symmetry allows only simple
crystal forms to be used by the Dolomite
Group minerals, mostly rhombohedrons.
Dolomite is a very common mineral and
ankerite is much more scarce. The other
members are considered rare to very
rare. The rarity of the members of this
group can be tied to the closeness in
radius of the A and B ions. In dolomite
the A and B ions are calcium and
magnesium which have the largest ionic
radius differential of the group
(approximately 33%). If the A and B ions
are close in radius, then they tend to
not segregate as easily into the
separate A and B layers, which is
required to form this structure and
therefore these minerals.
Recommended Filled of Application
| Kind of powder |
Talc |
Mica |
Kaolin |
Red Iron oxide |
Fluorine |
Dolomite |
Calcite |
Bentonite |
Barite |
| Ceramics |
• |
|
• |
• |
• |
• |
• |
• |
• |
| Chinaware |
• |
|
• |
|
|
|
|
|
|
| Excavation |
|
• |
|
|
|
• |
• |
• |
• |
| Elecrode |
• |
• |
|
|
• |
• |
• |
|
• |
| Feed |
|
|
|
|
|
|
• |
• |
|
| Glass |
• |
|
|
|
• |
• |
• |
|
• |
| Glaze |
|
|
• |
|
• |
• |
|
• |
|
| Glue |
• |
|
• |
|
|
|
• |
|
|
| Gerannlation (p.v.c) |
|
|
|
|
|
|
• |
|
|
| Insecticide |
• |
|
• |
|
|
|
|
• |
• |
| Isolation |
• |
|
|
|
|
|
• |
|
|
| lining |
|
|
|
|
|
• |
• |
|
• |
| Paint |
• |
• |
• |
• |
|
|
• |
• |
• |
| Pharmaceutical |
• |
|
• |
|
|
|
• |
• |
• |
| Plastic |
• |
|
• |
|
|
|
• |
|
• |
| Rulp & paper |
• |
|
|
|
|
|
• |
• |
• |
| Rubber |
• |
• |
• |
|
|
|
• |
|
• |
| Textile |
• |
|
|
|
|
|
• |
|
• |
|